On December 8, 2023, the U.S. Food and Drug Administration (FDA) approved the first-ever gene therapy that can treat patients with sickle cell disease, an inherited blood disorder. Vertex and CRISPR Therapeutics developed exagamglogene autotemcel or “exa-cel”, a gene therapy medication that targets the gene determining the shape and function of red blood cells. Sickle cell is a rare, debilitating, and life-threatening disease. In a healthy patient, the red blood cells are round and flexible, containing hemoglobin A (HbA), which carries oxygen to different parts of the body. Meanwhile, patients with sickle cell disease have long, polymerized sickle hemoglobin (Hbs), causing the red blood cells to distort their shape. The crescent-shaped red blood cells are less effective in delivering oxygen and tend to stick together blocking blood vessels.
Exa-cel can treat the mutated gene responsible for the development of sickle cell in an ex vivo process, where stem cells are taken from the body to be modified in the lab and infused back after gene editing. By using the CRISPR-Cas9 gene editing system, a specific red blood cell precursor gene in the bone marrow, known as the BCLL1A gene, is disrupted. Normally, BCLL1A tells the body to stop producing fetal hemoglobin. The goal of the treatment is to promote the production of fetal hemoglobin, which scientists believe can improve and lengthen the quality of life of sickle cell patients. The enzyme Cas9 acts like a pair of scissors which cuts into the gene to turn it off, allowing for fetal hemoglobin to be produced. With the CRISPR technology, scientists can identify the problematic genes and can install new or improved genes to treat diseases.
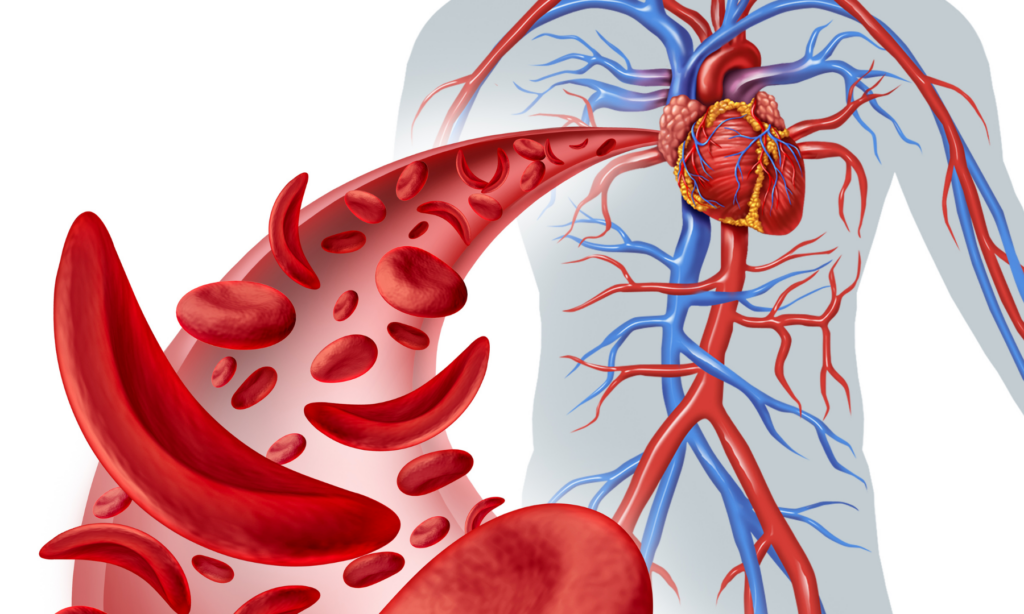
To date, exa-cel has only been tested in about 100 people with sickle cell disease or beta-thalassemia, both are genetic red blood cell disorders. There are currently two ongoing phase three trials taking place with promising results. There are 29 out of 30 sickle cell patients who received exa-cel reporting being pain-free for one year out of the 18 months since they were treated. Furthermore, 90% of beta-thalassemia patients no longer need blood transfusions within three years of receiving treatment. The target age group for these trials is between 12 to 35 years old, but there is a separate study investigating the effectiveness of the treatment in patients as young as two years old. A long-term study is also taking place, where scientists will observe patients who have received the infusion for the next 15 years. Based on current data, exa-cel has no major adverse complications but may come with side effects, such as nausea and fever.
The approval of exa-cel is shaping the future of gene-editing therapy. It has the power to change the lives of many patients suffering from inherited disorders. One of the major obstacles to making exa-cel available in the market is its inaccessibility. The treatment has been estimated to cost USD 2 million per patient. Since this is only the first step of many, companies and governments should prioritize making life-saving treatments such as exa-cel more accessible to the masses.
Roselle Torres
If you found the article interesting, I also recommend this:





